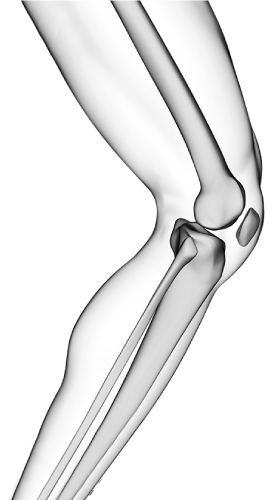

Asian Curvature
符合亞洲人骨型的亞洲曲率
愛派司始終以骨科醫師與病患的角度出發,
持續不斷地與台灣、亞洲各國學術界、臨床端合作, 專注於研發最適合亞洲人骨頭曲率的骨科創傷醫療器材,
堅持以最高的標準製造生產、提供最高品質的醫療器材內植入物,以最完善的專業知識、協助手術妥善進行,
提供亞洲患者舒適性的最佳治療。
205,718
外科醫生累計產品使用數量


符合亞洲人骨型的亞洲曲率
愛派司始終以骨科醫師與病患的角度出發,
持續不斷地與台灣、亞洲各國學術界、臨床端合作, 專注於研發最適合亞洲人骨頭曲率的骨科創傷醫療器材,
堅持以最高的標準製造生產、提供最高品質的醫療器材內植入物,以最完善的專業知識、協助手術妥善進行,
提供亞洲患者舒適性的最佳治療。
外科醫生累計產品使用數量